Explain How an Addition Polymer Differs From a Condensation Polymer
1 There are two types of polymerisation addition and condensation. N CH CH 3 CH CH 3 This polymer is made by addition polymerisation.

Difference Between Copolymer And Condensation Polymer Compare The Difference Between Similar Terms
Condensation polymerizations often but not always combine two different monomers in an alternating structure.

. Are proteins addition polymers or condensation polymers. Draw the repeating unit for a condensation polymer. An addition polymer is a polymer which is formed by an addition reaction where many monomers bond together via rearrangement of bonds without the loss of any atom or molecule.
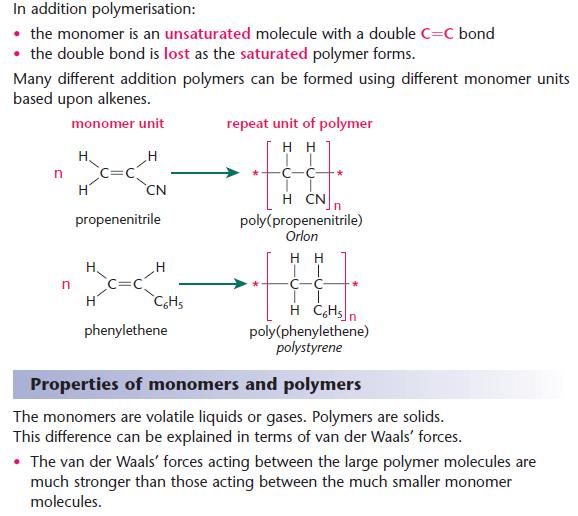
Addition polymerization is the process of repeated addition of monomers that possess double or triple bonds to form polymers. How do chiral molecules differ from each other. A condensation polymer is made of only one kind of monomer.
An addition polymer is made when monomers bond to each other without the loss of atoms. View Polymers 1 QPpdf from CHEM 104 at The Allied College of Education Gujranwala. The preferred terms today are chain growth and stepwise rather than addition and condensation.
An addition polymer is made when the monomers lose an atom or group of atoms while forming the polymer. AaThe structural formula of a polymer is given below. Textbook solution for Chemistry.
The main difference between addition and condensation polymerization is that in addition polymerization the polymers are formed by the addition of monomers with no by-products whereas in condensation polymerization the polymers are formed due to the condensation of more than one different monomers resulting in the formation of small. Chain growth involves monomer units reacting with each other in one direction. An addition polymer consists of two different kinds of monomers.
An addition polymer is made of only one kind of. A condensation polymer consists of two different kinds of monomers. This is called hydrolysis.
This means that condensation polymers are biodegradable and do not pose the same pollution hazard as addition polymers. A condensation polymer is formed when monomers bond to each other without the loss of atoms. The difference is in the mechanism of reaction.
The difference between addition and condensation polymerization is that for addition polymerization monomer should be an unsaturated molecule whereas for condensation polymerization. These linkages make the polymers reactive towards both acids and bases which cause the polymer structure to break down. H 2 N NH 2 HOOC.
Tro Chapter 20 Problem 34E. To form polyesters condensation polymerisation needs two types of monomer and forms two products. The main difference between addition polymerisation and condensation.
This is not the case for condensation polymerization wherein there is a chemical reaction between two or more monomer species producing the repeating unit. A condensation polymer is made of only one kind of monomer. Differentiate between the primary secondary tertiary and.
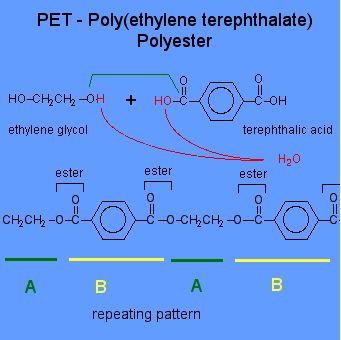
Ester links are formed and the polymer is called. Each monomer has two of the same functional groups. This is in contrast to a condensation polymer which is formed by a condensation reaction where a molecule usually water is lost during the formation.
Polymerization that occurs through the coupling of monomers using their multiple bonds and in other languages an addition polymer is a polymer that forms by simple linking of monomers without the co-generation of other products. We have step-by-step solutions for your textbooks written by Bartleby experts. Addition and condensation polymerization are the two major processes of producing a polymer compound.
Explain why condensation polymers are generally stronger and more rigid that addition polymers Condensation polymers are made up of chains containing polar bonds so as well as van der waals forces there are permanent dipole forces and. An addition polymer is made when the monomers lose an atom or group of atoms while forming the polymer. Condensation polymers can have molecular weights in the millions of Mgmol.
For addition polymerization the reactant species have the same chemical composition as the monomer species in the molecular chain. An addition polymer consists of two different kinds of monomers. 7 rows The main difference between addition and condensation polymerization is that in addition.
Draw a structural diagram of the linkage between amino acids in a peptide chain. Answer 1 of 9. 3 The molecular weight of the addition polymer is as low as 1-100000 Mgmol.
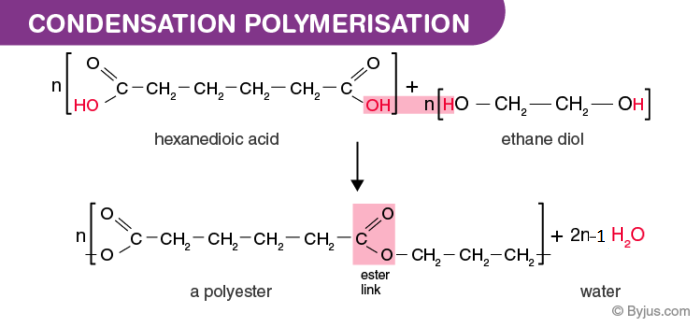
Condensation polymers all contain either the amide or ester linkages. Hexanedioic has two carboxylic acid groups. There is often a low molecular weight by-product for.
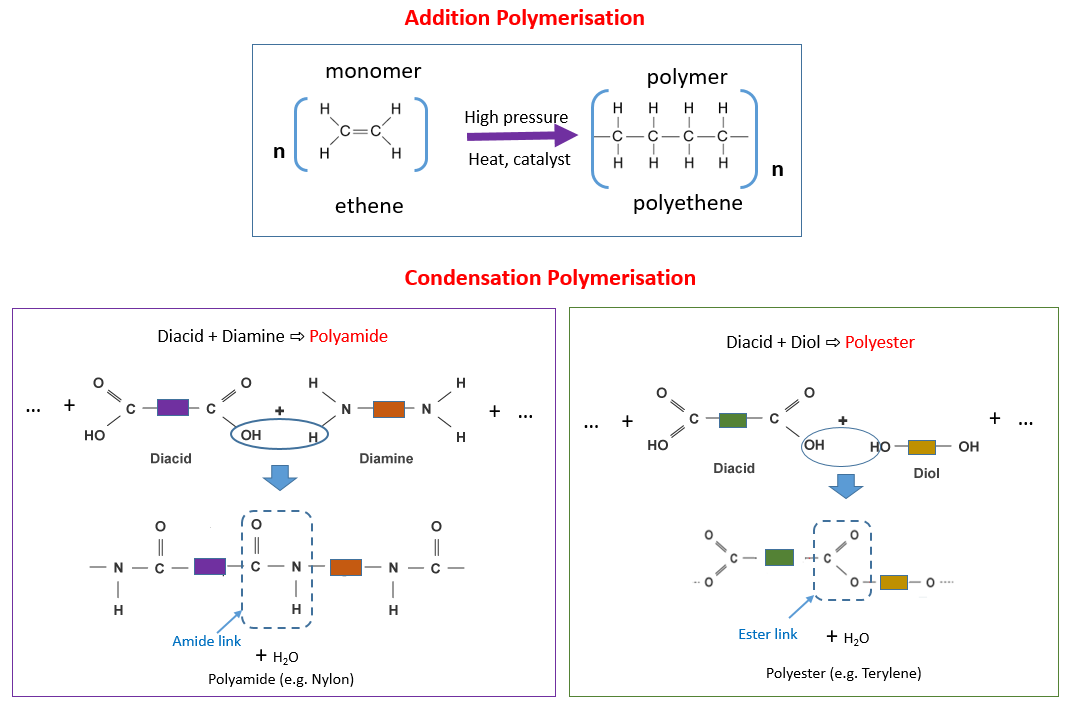
Addition polymerisation needs one type of monomer and forms one product. When these monomers react small molecules such as water are lost 2. Condensation polymerization is a process that involves repeated condensation reactions between two.
4 Polymers are made by the polymerisation of simple molecules called monomers. 14 rows Main Difference. A condensation polymer is made of only one kind of monomer.
A condensation polymer is a polymer formed via a condensation reaction. 1 Addition polymerization and 2 condensation polymerization. Draw the structural formula of its monomer.
The very small molecule produced in the condensation can be H 2 O HCl or some other simple molecule. 1 ii The two monomers shown below form a nylon which is a condensation polymer. The growing polymer has one active reaction site where.
An addition polymer is made of only one kind of monomer. An addition polymer is made of only one kind of. In addition polymers tend to form a complex structure that is thermodynamically unfavorable in comparison with condensation polymers tendency to form high molecular weight products.
Condensation polymers are made from two different monomers. From this table it must be quite obvious that the main difference between both addition and condensation polymerization is that in the case of addition polymerization the polymers are formed through the addition of monomers. A Molecular Approach 3rd Edition Nivaldo J.
Ethane diol has two alcohol groups. A condensation polymer is formed when monomers bond to each other without the loss of atoms. There are many differences between the two processes.

Addition Polymerization An Overview Sciencedirect Topics

Addition Polymerization And Condensation Polymerization Hindi Youtube
Condensation Polymers Examples Answers Activities Experiment Videos

Addition And Condensation Polymers Youtube
Polymers Chemistry A Level Revision

Distinguish Between Addition Polymers And Condensation Polymers Classify The Following Into Addition And Condensation Polymers Polythene Ptfe Polybutadiene And Bakelite Snapsolve
12 Difference Between Additional And Condensation Polymerization With Examples Viva Differences
Difference Between Addition Polymerisation And Condensation Polymerisation Process Features Type Of Polymers Produced Examples

Difference Between Addition And Radical Polymerization Compare The Difference Between Similar Terms

12 Difference Between Additional And Condensation Polymerization With Examples Viva Differences

Difference Between Addition Polymerization And Condensation Polymerization Compare The Difference Between Similar Terms

Nylon Is Made By Chemistry Questions

Nylon Is Made By Chemistry Questions
Condensation Polymerization Get Full Description Along With Examples
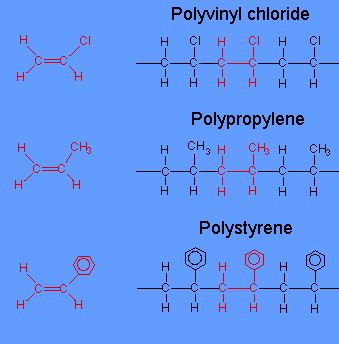
12 Difference Between Additional And Condensation Polymerization With Examples Viva Differences

Chemistry The Central Science Chapter 12 Section 2

Polymerization Reactions Chemistry Ii Types Of Polymerization Reactions Addition Polymerization Monomers Are Added Together With No Other Products Ppt Download

Condensation Polymers C 8 1 Distinguish Between Addition And Condensation Polymers In Terms Of Their Structures C 8 2 Describe How Condensation Polymers Ppt Download

Comments
Post a Comment